When your doctor talks about switching your medication to save money, you might hear the words biosimilar or generic. At first glance, they sound like the same thing: cheaper versions of expensive drugs. But they’re not. And mixing them up could cost you more than just cash-it could affect how well your treatment works.
What’s the Real Difference?
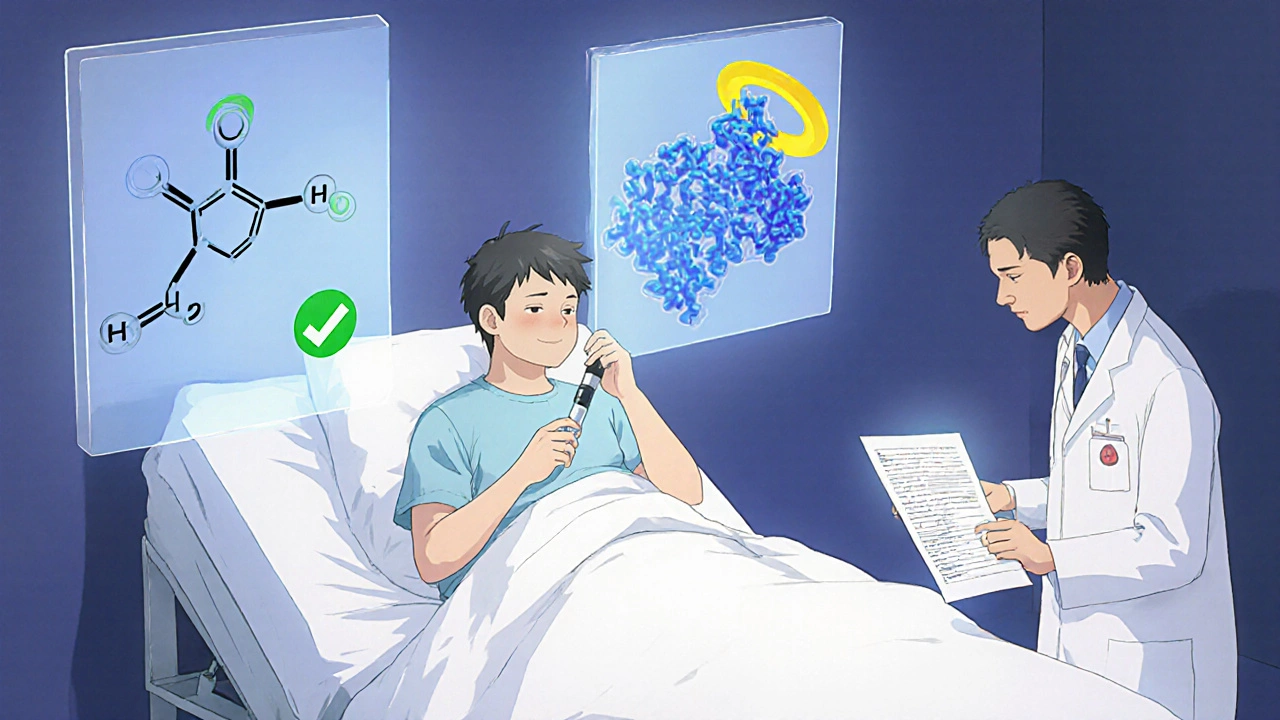
Generics and biosimilars both lower drug costs, but they come from completely different worlds of science.Generics are copies of small-molecule drugs-things like pills or capsules made from simple chemical formulas. Think of them like a photocopy of a handwritten note. The active ingredient is identical to the brand-name version. For example, the generic version of Lipitor is atorvastatin. It works the same way, in the same dose, with the same side effects. The FDA requires generics to prove they’re bioequivalent-meaning your body absorbs and uses them just like the original. That’s why over 90% of prescriptions in the U.S. are filled with generics today.
Biosimilars? They’re nothing like that. They’re copies of biologic drugs-complex proteins made from living cells. Think of them like trying to recreate a handmade sculpture using the same clay, tools, and artist. Even with the same instructions, no two sculptures are exactly alike. Biosimilars are highly similar to their reference drug, but not identical. They’re used for serious conditions like rheumatoid arthritis, Crohn’s disease, and cancer. Examples include adalimumab (Humira) and trastuzumab (Herceptin).
The difference isn’t just technical-it’s huge in cost. Generics can be 80-85% cheaper than the brand-name drug. Biosimilars? They’re only 15-20% cheaper. That’s because making a biologic drug is like running a living factory. It takes years, millions in equipment, and strict controls to grow cells that produce the protein. A generic drug might cost $2 million to develop. A biosimilar? $100 million to $250 million.
Why Can’t Biosimilars Be Called Generics?
You might wonder: if both are cheaper, why not just call them all generics? Because biology doesn’t work like chemistry.Generics are made in labs using chemical reactions. You can measure every atom. If the molecule matches, it’s the same drug. Biosimilars are grown in bioreactors using living cells-Chinese hamster ovary cells, yeast, or bacteria. Even tiny changes in temperature, nutrient mix, or pH can alter the final protein’s shape. And that shape? It matters. A slightly different fold in the protein can affect how your immune system reacts.
That’s why the FDA doesn’t just check a biosimilar’s chemical structure. They test it with advanced tools-mass spectrometry, chromatography, cell-based assays-to prove it behaves the same way in the body. They require clinical trials showing no meaningful difference in safety or effectiveness. But they can’t prove it’s identical. That’s why they’re called highly similar, not identical.
Who Gets Which One?
Not every drug has a biosimilar or a generic. And that shapes your options.If you’re taking a blood pressure pill, a statin, or thyroid medicine-you’re almost certainly on a generic. These are small molecules. There are over 11,000 approved generics in the U.S. And because they’re cheap and well-studied, pharmacists can swap them in automatically in 49 states unless your doctor says “dispense as written.”
Biosimilars? They’re only available for biologics-and those are mostly used for serious, chronic conditions. Think:
- Rheumatoid arthritis (adalimumab, etanercept)
- Psoriasis (ustekinumab, secukinumab)
- Colorectal cancer (bevacizumab)
- Breast cancer (trastuzumab)
- Diabetes (insulin glargine)
There are no generics for these drugs. You can’t chemically copy a protein that’s 15,000 times bigger than a typical drug molecule. That’s why biosimilars exist-they’re the only affordable option for these life-changing treatments.

Switching: Safe or Risky?
One of the biggest worries people have is switching from a brand-name biologic to a biosimilar. Will it stop working? Will you have more side effects?Studies say no. A 2022 review of 128 studies involving over 38,000 patients found no difference in safety or effectiveness between biosimilars and their reference drugs for infliximab and adalimumab. The American College of Rheumatology now recommends biosimilars as first-line treatment for rheumatoid arthritis. Cancer oncologists routinely switch patients to biosimilar versions of trastuzumab and rituximab with no drop in tumor response.
But there’s one exception: patients with inflammatory bowel disease (IBD). Some studies show a small uptick in anxiety after switching-even when lab results stay the same. That’s not because the drug changed. It’s because the fear of change is real. One patient on a cancer forum wrote: “My out-of-pocket dropped from $450 to $75 per infusion. My tumor markers didn’t budge.” Another with Crohn’s said: “I was scared. My doctor spent an hour explaining it. I’m fine now.”
For generics, switching is routine. A 2019 JAMA study of 47 trials found no difference in outcomes between brand-name and generic heart medications. Patients switch daily without thinking twice.
What About Interchangeability?
This is where things get legal.Not all biosimilars can be swapped at the pharmacy without your doctor’s permission. Only those labeled “interchangeable” can be substituted automatically. To earn that label, a biosimilar must prove it works just as well if you switch back and forth between it and the original drug.
As of 2025, only a handful of biosimilars have this status. The first interchangeable insulin (Semglee) hit the market in 2021. The first interchangeable anti-TNF drug (Cyltezo for Humira) followed in 2023. That means if your doctor prescribes Humira and you’re on an insurance plan that uses Cyltezo as interchangeable, your pharmacist can switch you without calling your doctor.
But here’s the catch: 28 states require the pharmacist to notify your doctor within 72 hours if they make that switch. And some doctors still don’t feel comfortable with automatic substitution. A 2023 AMA survey found only 58% of non-specialist doctors felt confident prescribing biosimilars. That’s down from 89% for generics.
Costs, Insurance, and Real-World Hurdles
Biosimilars save money-but not always for you.Insurance companies love biosimilars because they lower overall drug spending. But your copay might not reflect that. Some plans still charge you the same for a biosimilar as the brand-name drug. Others have high deductibles for specialty drugs, so you pay out of pocket until you hit your cap.
And getting a biosimilar approved can be a mess. A 2022 study found 67% of biosimilar prior authorizations needed extra paperwork-compared to 42% for generics. That means delays. Missed doses. Frustration.
Meanwhile, generics are simple. Your pharmacy has them in stock. Your insurance covers them. You pay $5. No forms. No waiting.
There’s also a hidden cost: education. Many patients don’t understand the difference. A 2022 survey by the National Psoriasis Foundation found 42% of patients were worried biosimilars wouldn’t work. One Reddit user wrote: “My insulin pen looks different. I was afraid I’d mess up the dose.” That’s why manufacturer support programs-like Amgen’s SupportPlus-are growing. They offer nurse hotlines, injection training, and financial aid.
What Should You Do?
If you’re considering a switch, here’s what to ask:- Is my drug a biologic or a small molecule?
- Is there a generic version available? (If yes, it’s almost always the best choice.)
- If it’s a biologic, is there a biosimilar? Is it FDA-approved? Is it interchangeable?
- What’s my out-of-pocket cost with each option?
- Has my doctor explained why this switch is safe?
- Will my insurance cover it without a long approval process?
Don’t assume cheaper means worse. Biosimilars aren’t “second-rate.” They’re backed by years of testing, real-world data, and regulatory rigor. But they’re not magic. They’re science-and science needs understanding.
If you’re on a biologic and your doctor suggests a biosimilar, ask for a printed handout. Request a 15-minute chat. Ask: “What’s the evidence this will work for me?” Most doctors will be happy to explain.
And if you’re on a generic? Don’t second-guess it. You’re getting the same drug, for a fraction of the price. Generics have saved the U.S. healthcare system over $300 billion in the last decade. That’s not luck. That’s science working.
Final Thought: It’s Not About Cheap-It’s About Smart
Choosing between a biosimilar and a generic isn’t about picking the lowest price. It’s about matching the right tool to the right problem.For high blood pressure? Generic. Always.
For rheumatoid arthritis or cancer? Biosimilar-when available-is a breakthrough. It’s the only way millions can afford treatment.
Both options are safe. Both are proven. The difference is in the science, not the safety. Your job? Ask questions. Understand your options. And don’t let fear stop you from getting the care you need.
9 Comments
Man i just found out my rheumatoid arthritis med is a biosimilar and i was terrified at first but my doc sat me down and showed me all the studies
turns out my bloodwork is better now and i pay like 30 bucks a month instead of 500
why do people think cheaper means worse? it’s not magic, it’s science
It’s fascinating how society conflates cost-efficiency with inferiority, particularly in pharmaceuticals
The epistemological gap between chemical synthesis and biologic replication is not merely technical-it’s ontological
When we label biosimilars as ‘similar’ rather than ‘identical,’ we are not admitting scientific uncertainty-we are acknowledging the inherent variability of life itself
Yet the market demands binary categorization: generic = good, brand = trusted
This is not rational-it’s cultural
The FDA’s regulatory framework is remarkably sophisticated, yet public perception remains rooted in the myth of chemical purity
Perhaps the real crisis isn’t drug efficacy-it’s our inability to trust complexity
Hey i just want to say thank you for writing this
as someone who’s been on Humira for 8 years and switched to Cyltezo last year-i was nervous as hell
but my insurance pushed it and my doc said ‘it’s basically the same but cheaper’
turns out i didn’t even notice a difference
and now i can afford to take my kid to the zoo without selling a kidney
also the nurse who helped me with the injection? she was a saint
ps: if you’re scared, ask for the support program
they’ll walk you through it
so like i just got switched to a biosimilar for my psoriasis and at first i was like oh no not again
but then i read your post and i was like… wait this makes sense
generics are like copying a song on your phone
biosimilars are like trying to recreate the exact same live concert with a different band
it’s not the same but it still gives you chills
and my out of pocket went from $400 to $80
so yeah i’m chill now
also i misspelled a bunch of words here because i’m typing on my phone and i’m tired
Coming from Ireland, we’ve had biosimilars for years and honestly? no one bats an eye
my mate switched from Remicade to a biosimilar and his arthritis got better
the system here just assumes if it’s approved, it’s good
we don’t have this whole ‘brand trust’ thing like in the US
also the pharmacist just handed me the new pen and said ‘same thing, cheaper’
no forms, no drama
why can’t we just be like this?
This is such an important post ❤️
I’ve been on insulin for 15 years and just switched to Semglee last month
My copay dropped from $120 to $15
I cried in the pharmacy because I finally felt like I could breathe again
Thank you for explaining the science so clearly
Don’t let fear stop you from saving your life
You’re not taking a risk-you’re choosing hope
my mom is on a biosimilar for Crohn’s and she was convinced it wouldn’t work
she kept saying ‘it looks different’ and ‘what if it fails?’
so i printed out the FDA’s comparison charts and we watched a 10-minute video together
now she’s like ‘ohhh so it’s just like a generic but for fancy proteins?’
and she’s been fine for 6 months
the real enemy isn’t the drug-it’s the silence
talk to your doc. ask questions. it’s okay to be scared
but don’t stay scared
just read this whole thing
thank you
my brother is on trastuzumab and they switched him to a biosimilar last year
he thought he was gonna die
turns out he’s been in remission longer than before
the only difference? his wallet
also i typoed like 5 times while typing this
but you get the point
my pharmacist switched my generic blood pressure med without telling me and i didn’t even notice
it’s just a pill
why is this so hard to believe for biologics?
Write a comment